© Copyright 2020 Foshan Membrane Technology Co., Ltd. All rights reserved. Sitemap
The existing 3D-printed scaffolds commonly possess a thick feature size of hundreds of micrometers, which is too large for most cells (10–20 μm) to attach and proliferate for promoting tissue regeneration. Researchers from Xi’an Jiaotong University have developed a novel hybrid manufacturing technique for the fabrication of composite scaffolds with 3D-printed macroscale frameworks and aligned nanofibrous architectures to improve cellular organizations.
Publishing in the journal International Journal of Extreme Manufacturing, the team led by researchers based at State Key Laboratory for Manufacturing Systems Engineering combined the techniques of 3D printing, electrospinning, unidirectional freeze-casting, and lyophilization to embed ECM-biomimetic fibrillar architectures inside previously 3D-printed scaffolds. Compared with 3D-printed scaffolds, the developed composite scaffolds with hierarchical structures were able to improve the seeding efficiency, proliferation rate, and morphogenesis of the seeded cells, and guide the directional cellular ingrowth. The findings could have a widespread impact on the development of composite scaffolds with hierarchical architectures potentially for the orderly spatial regeneration and remodeling of tissues in the future.
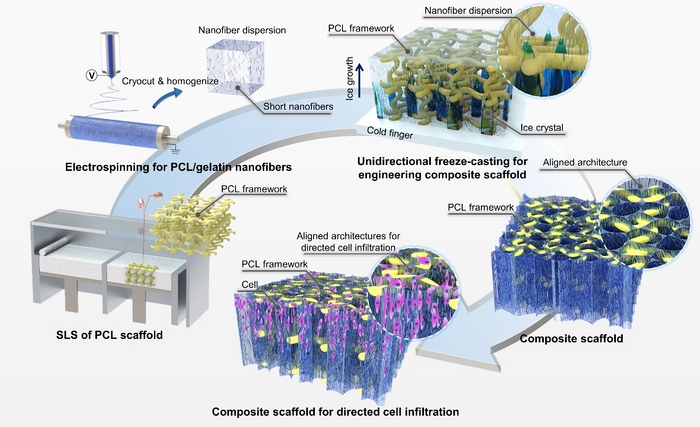
IMAGE: COMPOSITE SCAFFOLDS WITH LOAD-BEARING FRAMEWORKS AND ALIGNED NANOFIBROUS ARCHITECTURES WERE HYBRID MANUFACTURED BY COMBINING TECHNIQUES OF 3D PRINTING, ELECTROSPINNING, UNIDIRECTIONAL FREEZE-CASTING, AND LYOPHILIZATION. IN THE COMPOSITE SCAFFOLDS, THE 3D-PRINTED FRAMEWORKS PROVIDED SUFFICIENT MECHANICAL STRENGTH FOR COUNTERACTING BIOLOGICAL LOADS IN VIVO, WHILE THE EMBEDDED NANOFIBROUS ARCHITECTURES WITH UNIDIRECTIONAL MICROPORES PROVIDED ADDITIONAL SITES AND GUIDANCE FOR DIRECTED CELLULAR INFILTRATION.
One of the lead researchers, Professor Jiankang He, commented, “The emergence of 3D printing technologies has enabled the rapid and customized fabrication of porous scaffolds with designer structural and mechanical properties, exhibiting great potential for various tissue repairing applications and future clinical usage. Nevertheless, one of the challenges of current 3D-printed scaffolds is the relatively large feature size, which limited the cell attachment and growth for the formation of dense cellular constructs for promoting tissue reconstruction. Given the widespread medical and scientific importance of 3D printing, it is truly important to enhance the capability of 3D-printed scaffolds to meet the pressing needs of facilitating tissue regeneration. One of the promising directions is to incorporate additional micro/nanoscale architectures inside the macroscale 3D-printed scaffolds as ECM alternatives for cellular colonization, organization, and maturation.”
“Currently, few techniques can be utilized to introduce collagen-like micro/nanofibers within existing porous scaffolds due to the shielding effects of the existing architectures.” First author Dr. Zijie Meng said, “In our work, we show that ECM-mimetic fibrillar architectures could be incorporated into the 3D-printed scaffolds by freeze-casting the perfused short nanofiber suspensions into solid and then remove the ice via freeze-drying. Nanofibrillar architectures with aligned orientation can be obtained under the guidance of a unidirectional temperature gradient, which might be useful for promoting infiltration and migration of surrounding cells. By changing the freezing temperature, the median pore area of the nanofibrous architectures can be further controlled from c.a. 400 μm2 to 4000 μm2.”
This novel combination allowed them to produce additional topological cues within mechanically-robust 3D-printed scaffolds. By seeding cells on the composite scaffolds with aligned nanofibrous architectures in vitro, researchers were able to understand the effect of the pore size of the aligned nanofibrillar architectures on cellular attachment, proliferation, and directed infiltration. The existence of nanofibrous architectures was found to significantly improve the cell seeding efficiency, proliferation rate, and directed cellular migration, as compared with pure 3D-printed scaffolds with large pore sizes and thick filaments.
Co-first author Miss Xingdou Mu at the Air Force Medical University added, “The composite scaffolds can provide volume-stable environments, enable directed cellular infiltration for tissue regeneration, and support the adipogenic maturation of ADSCs in vitro. Especially, the 3D-printed frameworks provided the majority of the mechanical support capacity of the composite scaffolds, while the cellular responses were found to be directly influenced by the embedded nanofibrous architectures. Additionally, when implanted into a subcutaneous model of rats, the composite scaffolds with aligned nanofibrous architectures can guide directed tissue infiltration and effectively promote nearby neovascularization, which might be helpful for the long-term survival of the regenerated tissues.”
The team studied a hybrid manufacturing strategy that is promising for composite scaffold production with hierarchical structures, and the experimental technology they have developed can be used for many different applications. Co-corresponding author Professor Juliang Zhang said, “The host tissues were able to gradually infiltrate into the composite scaffolds along the direction of aligned nanofibrous structures, with the 3D-printed PCL frameworks contributing to the shape retention of the regenerated tissues. In the future, the feasibility to arrange cellular organization by changing the local orientation of nanofibrous micropores need further and deeper investigation, which might be potentially used for more complex and aligned tissue regeneration such as the tendon, ligament, nerve, and cardiac muscles.”
Article Source:https://www.eurekalert.org/news-releases/982665